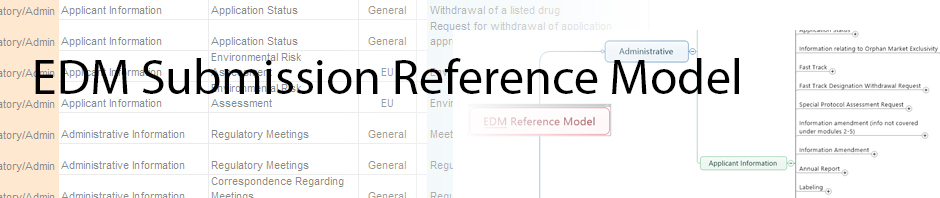
The GMP Quality Systems reference model linked below has been finalized and is awaiting posting to the DIA public website.
The model is intended to be a reference for the artifacts, taxonomy and document properties (metadata) needed to manage documents required for GMP (Good Manufacturing Practices) compliance (e.g., SOPs, policies, specifications, methods, etc.). This model is a reference for the life science industry and should not be considered mandatory, but rather as an opportunity for standardization across the industry to enable greater interoperability and simplify business process hand-offs among sponsors, business partners, and suppliers / service providers (e.g., contract manufacturers) related to GMP documents and content required for regulatory compliance. Look for software vendors that incorporate this model as a starting point taxonomy and metadata model for their customers to organize their GMP compliance content as they configure or upgrade their quality content management systems.
We appreciate all the input provided by our colleagues in the Quality and Regulatory space to enable us to complete Version 1.0 of this reference model as well as the many hours the team who participated in meetings over the last 2 years spent to achieve this milestone. We are excited to see how the model gets used in the Life Sciences industry and look forward to receiving feedback on ways to improve it over time.
https://edmrefmodel.com/download/dia-gmp-quality-systems-reference-model-v1-0/DIA GMP Quality Systems Reference Model v1.0