Announcing the DIA EDM Structured Submission Reference Model
2023-01-18 12:11:06We have come a long way since the first version of the EDM Submission Reference Model was drafted in 2009. With an eye on the future, the model was designed to be independent of any single software solution and to allow for growth and implementation by any software platform that should evolve. It was intended to be flexible and adaptable by both sponsors and vendors, and it is exciting to see the number of vendors acknowledging their alignment with the model today.
As the years progressed, Health Authority regulations have changed, and new requirements have become law, but the way content is authored, reviewed and approved has evolved very little in the past 14 years. We still create Company Core Data Sheets (CCDS) and pass them to affiliates to use when creating local labels, playing a never-ending cycle of, “What did they submit?”. We still craft the same product description presented in countless documents and hopefully cut-and-pasted correctly without someone adding “just a few updates.” We manually create drug substance or drug product specifications that are pages of tables of core data that could be created automatically with the technology available today. We still translate the same content multiple times, paying again for the same content. Isn’t it time for a change? As the saying goes, “If we always do what we always did, we will always get what we always got.”
With the world still reeling from the impact of a global pandemic, the old mantra of “Reducing time to market” for new drugs, biologics and devices is more poignant than ever. Shrinking the time from the last patient out to the release of a global submission has many moving parts. However, the management and product of documents and related content is still a critical element of that timeline. Imagine a scenario where the authors of the final clinical study report are presented with a collection of approved content that can be rapidly glued together instead of written from scratch. At the same time, authors in the Quality area review the final specification documents created automatically through XML-driven templates rather than starting from a blank document. Consider the time and cost savings of having the CCDS sections individually approved and translated, ready for all global partners to use immediately.
Once the initial marketing applications are approved, the lifecycle of maintaining those approvals begins. Consider a scenario where a change to the safety section of the CCDS is required, and corporate can instantly assess the global impact because each element of the CCDS is broken down into smaller parts, and its use in local labels is tracked. Further, once that section is updated in the CCDS, only the changed text needs to be translated. How much money could be saved on printing labels and packaging materials, knowing what changes are in development and when they are expected to be approved?
All of these scenarios are possible today. The tools have matured to allow users to create structured content without needing a degree in XML authoring. Component content management systems (CCMS) are robust and capable of delivering the elements to the desktop authoring solutions in meaningful and useful ways, providing the means to author structured documents while still focusing on the science and not the mechanics of crafting a document.
When we started this journey in 2009, we had content management solutions (CMS) like Documentum and authoring platforms like Word (and maybe even WordPerfect). But none of these solutions “knew” the difference between a toxicology report or a jet engine turbine. They were blank slates that needed configuration to support the authoring process by telling the CMS what objects to manage and providing templates with headings to the authoring tools. That was why the original reference model was created – to provide a starting point for these tools upon which a solution could be built and accelerate deployments across the industry.
The cycle needs to be repeated. This time we have CCMS platforms and Word-like structured authoring tools to create our content. But how do we break down a CCDS into smaller parts that make sense? What parts of documents can be reused in other documents? What documents can be created automatically from the data we manage so carefully in our systems today? The purpose of this group is to address these questions, as we did 14 years ago, to provide a new way of handling the information we have been managing for decades.
Please join us on 14-Feb-2023 at 5:30 PM – 6:00 PM in the Linen Oak room at the conference hotel for an introductory session to kick off this important initiative. Everyone is welcome to join, and we look forward to an exciting discussion.
Latest Draft of EDM Sub Reference Model
2016-03-03 12:45:12The latest draft of the EDM Submission Reference Model is available for review. This includes the latest content regarding prescribing information and also updates to the Regulatory / Administrative information. It can be downloaded at the link below:
https://edmrefmodel.com/download/edm-submission-reference-model-20-draft/
Integration with TMF Reference Model
2015-01-07 11:24:38Today’s agenda for the EDM Submission Reference Model team includes a discussion on the overlap and integration between the EDM Submission and TMF Reference models. While the TMF model was spawned from the EDM team a number of years ago, the two models have evolved over time independently. We have discussed as a team the need for reviewing the overlaps and ensuring we are aligned between the two models.
An initial assessment has uncovered several areas that may be overlapping as shown in the table below:
| TMF Ref Model | EDM Ref Model |
| Site Signature Sheet | Signature of PI or Sponsor |
| Interim Analysis Output Interim Analysis Raw Datasets Interim Analysis Program Interim Analysis Datasets |
Documentation of Statistical Methods and Interim Analysis |
| Data Definitions for Analysis Datasets | Clinical – CSR – Datasets – Analysis Datasets – Data Definitions |
| PK Report | Clinical – Human PK Studies – eCTD 5.3.3.1 to 5.3.3.5 |
| Site Management – Protocol Deviation | Clinical – CSR – Appendices – Data Listings – Protocol Deviation |
| Financial Disclosure Form | Financial Disclosure Cover Note |
| Regulatory Meeting | Administrative – Administrative Information – Regulatory Meetings – General:
– Regulatory Meeting Agenda – Meeting Background Materials – Regulatory Meeting Presentation – Correspondence Regarding Meetings |
| CSR Synopsis | Clinical – CSR – Synopsis |
Todays’ meeting will discuss an approach to addressing these potential overlapping areas.
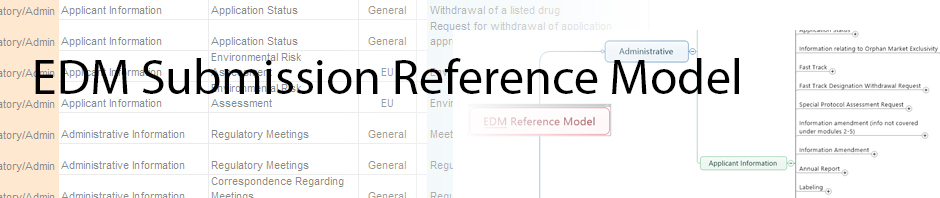
Mind Map of Reference Model
2015-01-05 20:27:20In an effort to modernize our delivery of reference model information, the team used techniques that we use in content management projects which include mind mapping techniques for visualization.
We have created a [wpdm_hotlink id=’88’ link_label=’mind map’] of the model available for download. The file was created using MindJet’s Mind Manager, however the file can be viewed and navigated without access to the software. All that is required is a PDF viewing tool.