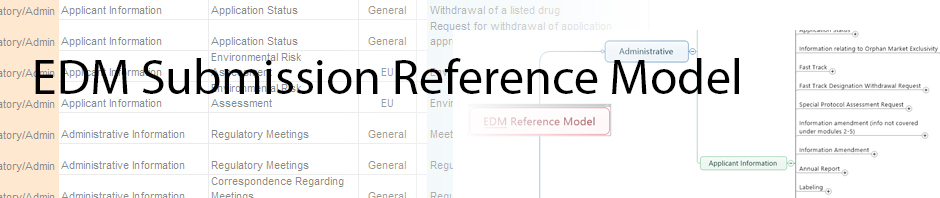
The Electronic Document Management (EDM) Reference Model is intended for biopharmaceutical companies implementing an EDM system or a simple file share to store and prepare documents for regulatory submissions of drugs and biologic products intended for human use. While the model provides the content, a Regulatory Publishing system is still required to assemble and publish a true regulatory submission. The Reference Model is also useful for biopharmaceutical companies needing to organize and transfer documentation assets of a product to another company’s repository under an acquisition or partnership scenario. In this case, the Reference Model provides the common document definition and structure that both companies can share.
Meeting Calendar
Events in July 2024
MMonday TTuesday WWednesday TThursday FFriday SSaturday SSunday 11-Jul-202422-Jul-202433-Jul-202444-Jul-202455-Jul-202466-Jul-202477-Jul-202488-Jul-202499-Jul-20241010-Jul-20241111-Jul-20241212-Jul-20241313-Jul-20241414-Jul-20241515-Jul-20241616-Jul-20241717-Jul-20241818-Jul-20241919-Jul-20242020-Jul-20242121-Jul-20242222-Jul-20242323-Jul-20242424-Jul-20242525-Jul-20242626-Jul-20242727-Jul-20242828-Jul-20242929-Jul-20243030-Jul-20243131-Jul-202411-Aug-202422-Aug-202433-Aug-202444-Aug-2024-
Recent Posts
Recent Comments
- M. Rogers on Latest Draft of EDM Sub Reference Model
- Thierry Felix on Latest Draft of EDM Sub Reference Model
- Silvio Mann on Latest Draft of EDM Sub Reference Model
- Carl Sampson on Latest Draft of EDM Sub Reference Model
- Romuald on Device Reference Model Team Formed
Archives
Meta