Meeting Calendar
Events in May 2024
MMonday TTuesday WWednesday TThursday FFriday SSaturday SSunday 2929-Apr-20243030-Apr-202411-May-202422-May-202433-May-202444-May-202455-May-202466-May-202477-May-202488-May-202499-May-20241010-May-20241111-May-20241212-May-20241313-May-20241414-May-20241515-May-20241616-May-20241717-May-20241818-May-20241919-May-20242020-May-20242121-May-20242222-May-20242323-May-20242424-May-20242525-May-20242626-May-20242727-May-20242828-May-20242929-May-20243030-May-20243131-May-202411-Jun-202422-Jun-2024-
Recent Posts
Recent Comments
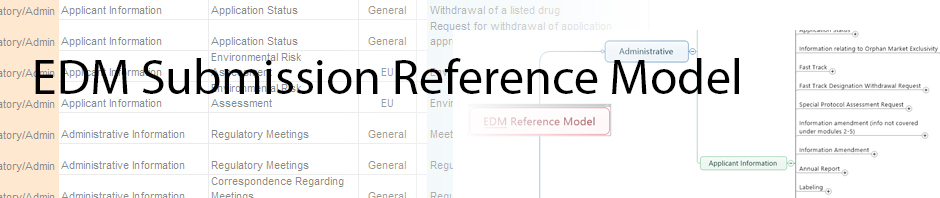
- M. Rogers on Latest Draft of EDM Sub Reference Model
- Thierry Felix on Latest Draft of EDM Sub Reference Model
- Silvio Mann on Latest Draft of EDM Sub Reference Model
- Carl Sampson on Latest Draft of EDM Sub Reference Model
- Romuald on Device Reference Model Team Formed
Archives
Meta
Device Ref Model Forum
Back to Device Ref Model Forum...
Dear,
Please find enclosed Meeting minutes.
Regards,
Metod
(if any)
Note: to be in line with the content we’ve changed the naming for previous sheets:
M. Ostanek – done
•Clinical (includes also non-clinical attributes)