Meeting Minutes - 21.May.2015 (1 reply and 1 comment)
Link to minutes in Word: http://1drv.ms/1PZEFxG»
Link to meeting recording (WEBEX Network Recording Player required): http://1drv.ms/1PZEEtD»
WEBEX palyer download: http://www.webex.com/play-webex-recording.html»
(if any)
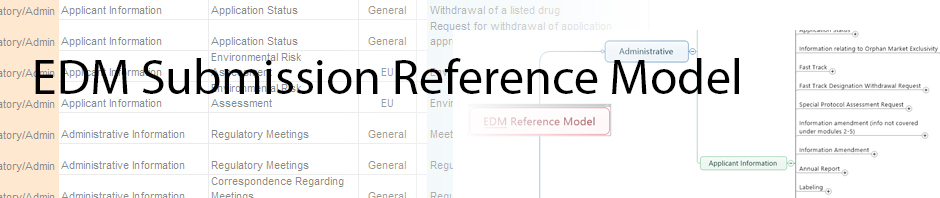
•Prescribing Information /Labeling,
•CMC /Technical/Quality & Mnf/
•Clinical (includes also non-clinical attributes)
-MEDTECHEUROPE
(See links below)