Meeting Calendar
Events in April 2024
MMonday TTuesday WWednesday TThursday FFriday SSaturday SSunday 11-Apr-202422-Apr-202433-Apr-202444-Apr-202455-Apr-202466-Apr-202477-Apr-202488-Apr-202499-Apr-20241010-Apr-20241111-Apr-20241212-Apr-20241313-Apr-20241414-Apr-20241515-Apr-20241616-Apr-20241717-Apr-20241818-Apr-20241919-Apr-20242020-Apr-20242121-Apr-20242222-Apr-20242323-Apr-20242424-Apr-20242525-Apr-20242626-Apr-20242727-Apr-20242828-Apr-20242929-Apr-20243030-Apr-202411-May-202422-May-202433-May-202444-May-202455-May-2024-
Recent Posts
Recent Comments
- M. Rogers on Latest Draft of EDM Sub Reference Model
- Thierry Felix on Latest Draft of EDM Sub Reference Model
- Silvio Mann on Latest Draft of EDM Sub Reference Model
- Carl Sampson on Latest Draft of EDM Sub Reference Model
- Romuald on Device Reference Model Team Formed
Archives
Meta
TMF Tech Presentation Group
Back to TMF Tech Presentation Group...
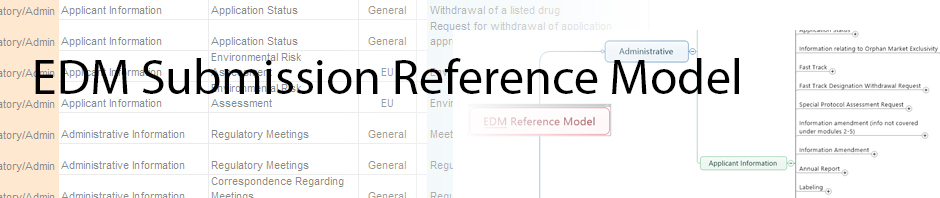
During our last meeting the question was raised regarding the purpose of our efforts - what user requirements did we have that we were trying to address? This question spawned terrific dialog which lead us to the realization that we have not yet documented the true user requirements. The list below was provided Eldin from feedback received from the model. Hopefully we can cull through this list and use it to identify user requirements for the presentation group. Please feel free to put requirements you see in this list or others you define in response to this thread.